Entamoeba histolytica
|
Entamoeba
histolytica |
|
|
Entamoeba
histolytica trophozoite |
|
|
Domain: |
|
|
Phylum: |
|
|
Family: |
|
|
Genus: |
|
|
Species: |
E. histolytica |
|
Entamoeba
histolytica |
|
.0.0
Entamoeba
histolytica is an anaerobic parasitic amoebozoan, part of
the genus Entamoeba. Predominantly
infecting humans and other primates causing amoebiasis, E.
histolytica is estimated to infect about 35-50 million people
worldwide. E.histolytica infection
is estimated to kill more than 55,000 people each year. Previously, it was
thought that 10% of the world population was infected, but these figures
predate the recognition that at least 90% of these infections were due to a
second species, E. dispar. Mammals such as dogs and cats
can become infected transiently but are not thought to contribute significantly
to transmission.
The word histolysis literally
means disintegration and dissolution of organic tissues.
Transmission
The active (trophozoite) stage exists
only in the host and in fresh loose feces; cysts survive
outside the host in water, in soils, and on foods, especially under moist
conditions on the latter. The infection can occur when a person puts anything
into their mouth that has touched the feces of a person who is infected
with E. histolytica, swallows something, such as water or food,
that is contaminated with E. histolytica, or swallows E.
histolytica cysts (eggs) picked up from contaminated surfaces or
fingers. The cysts
are readily killed by heat and by freezing temperatures; they survive for only
a few months outside of the host.[5] When cysts
are swallowed, they cause infections by excysting (releasing the trophozoite
stage) in the digestive tract. The pathogenic nature of E. histolytica was
first reported by Fedor A. Lösch in 1875, but it was
not given its Latin name until Fritz Schaudinn described
it in 1903. E. histolytica, as its name suggests (histo–lytic =
tissue destroying), is pathogenic; infection can
be asymptomatic, or it can lead to amoebic
dysentery or amoebic liver abscess. Symptoms
can include fulminating dysentery, bloody diarrhea, weight loss, fatigue,
abdominal pain, and amoeboma. The amoeba can
'bore' into the intestinal wall, causing lesions and intestinal symptoms, and
it may reach the blood stream. From there, it can reach vital organs of the
human body, usually the liver, but sometimes the lungs, brain, and spleen. A
common outcome of this invasion of tissues is a liver abscess, which can be
fatal if untreated. Ingested red blood cells are
sometimes seen in the amoeba cell cytoplasm.
Risk factors
Poor sanitary
conditions are known to increase the risk of contracting amebiasis E.
histolytica.
In the United States, there is a much higher rate
of amebiasis-related mortality in California and Texas (this might be caused by
the proximity of those states to E. histolytica-endemic areas, such
as Mexico), parts of Latin America, and Asia. E.
histolytica is also recognized as an emerging sexually transmissible
pathogen, especially in male homosexual relations, causing outbreaks in
non-endemic regions. As such, high-risk sex
behavior is also a potential source of infection. Although it
is unclear whether there is a causal link, studies indicate a higher chance of
being infected with E. histolytica if one is also infected
with HIV.
Genome
The E.
histolytica genome was
sequenced, assembled, and automatically annotated in 2005. The genome
was reassembled and reannotated in 2010.[15] The 20
million basepair genome assembly contains 8,160 predicted genes known and
novel transposable elements have been
mapped and characterized, functional assignments have been revised and updated,
and additional information has been incorporated, including metabolic
pathways, Gene Ontology assignments,
curation of transporters, and generation of gene families.[16] The major
group of transposable elements in E. histolytica are non-LTR
retrotransposons. These have been divided in three families called EhLINEs and
EhSINEs (EhLINE1,2,3 and EhSINE1,2,3). EhLINE1 encode an endonuclease
(EN) protein (in addition to reverse transcriptase and nucleotide-binding
ORF1), which have similarity with bacterial restriction endonuclease. This similarity with bacterial
protein indicates that transposable
elements have been acquired from prokaryotes by horizontal gene transfer in this
protozoan parasite.
The genome
of E. histolytica has been found to have snoRNAs with opisthokont-like features. The E. histolytica U3 snoRNA
(Eh_U3 snoRNA) has showed sequence and structural features similar to Homo
sapiens U3 snoRNA.
Pathogen interaction
E. histolytica may
modulate the virulence of certain human viruses and is itself a host for its
own viruses.
For example, AIDS
accentuates the damage and pathogenicity of E. histolytica.[13] On the
other hand, cells infected with HIV are often consumed by E.
histolytica. Infective HIV remains viable within the amoeba, although there
has been no proof of human reinfection from amoeba carrying this virus.
A burst of
research on viruses of E. histolytica stems from a series of
papers published by Diamond et al. from 1972 to 1979. In 1972,
they hypothesized two separate polyhedral and filamentous viral strains
within E. histolytica that caused cell lysis. Perhaps the most
novel observation was that two kinds of viral strains existed, and that within
one type of amoeba (strain HB-301) the polyhedral strain had no detrimental
effect but led to cell lysis in another (strain HK-9). Although Mattern et al.
attempted to explore the possibility that these protozoal viruses could
function like bacteriophages, they found no significant changes in Entamoeba
histolytica virulence when infected by viruses.
Immunopathogenesis
E. histolytica causes
tissue destruction which leads to clinical disease. E. histolytica–induced
tissue damage by three main events: direct host cell death, inflammation, and
parasite invasion. Once the trophozoites are excysted in the terminal ileum
region, they colonize the large bowel, remaining on the surface of the mucus
layer and feeding on bacteria and food particles. Occasionally, and in response
to unknown stimuli, trophozoites move through the mucus layer where they come
in contact with the epithelial cell layer and start the pathological
process. E. histolytica has a lectin that binds
to galactose and N-acetylgalactosamine sugars on the surface of the epithelial
cells, The lectin normally is used to bind bacteria for ingestion. The parasite
has several enzymes such as pore forming proteins, lipases, and cysteine
proteases, which are normally used to digest bacteria in food vacuoles but
which can cause lysis of the epithelial cells by inducing cellular necrosis and
apoptosis when the trophozoite comes in contact with them and binds via the
lectin. Enzymes released allow penetration into intestinal wall and blood
vessels, sometimes on to liver and other organs. The trophozoites will then
ingest these dead cells. This damage to the epithelial cell layer attracts
human immune cells and these in turn can be lysed by the trophozoite, which
releases the immune cell's own lytic enzymes into the surrounding tissue,
creating a type of chain reaction and leading to tissue destruction. This
destruction manifests itself in the form of an 'ulcer' in the tissue, typically
described as flask-shaped because of its appearance in transverse section. This
tissue destruction can also involve blood vessels leading to bloody diarrhea,
amebic dysentery. Occasionally, trophozoites enter the bloodstream where they
are transported typically to the liver via the portal system. In the liver a
similar pathological sequence ensues, leading to amebic liver abscesses. The
trophozoites can also end up in other organs, sometimes via the bloodstream,
sometimes via liver abscess rupture or fistulas. Similarly, when
the trophozoites travel to the brain, they can cause amoebic brain abscess.
Diagnosis
Diagnosis is
confirmed by microscopic examination for trophozoites or cysts in fresh or
suitably preserved faecal specimens, smears of aspirates or scrapings obtained
by proctoscopy, and aspirates of abscesses or other tissue specimen. A blood
test is also available, but it is recommended only when a healthcare provider
believes the infection may have spread beyond the intestine to some other organ
of the body, such as the liver. However, this blood test may not be helpful in
diagnosing current illness, because the test can be positive if the patient has
had amebiasis in the past, even if they are not infected at the time of the
test.[24] Stool
antigen detection and PCR are available for diagnosis, and are more sensitive
and specific than microscopy.
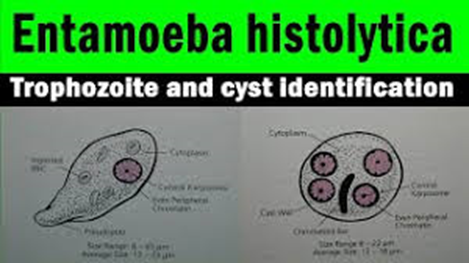
Entamoeba histolytica trophozoite
Amoebic intestinal ulcer caused by E.
histolytica
Trophozoites of E.
histolytica with ingested erythrocytes
E. histolytica cyst
Immature E. histolytica cyst (mature
cysts have 4 nuclei)
E. histolytica quadrinucleate cyst with
chromatoid bodies.
Multiplication by binary fission
E. histolytica drawing
Immunohistochemical staining of trophozoites (brown)
using specific anti–Entamoeba histolytica macrophage migration
inhibitory factor antibodies in a patient with amebic colitis.
Treatment
There are a
number of effective medications. Several antibiotics are available to
treat Entamoeba histolytica. The infected individual will be
treated with only one antibiotic if the E. histolytica infection
has not made the person sick, and will most likely be prescribed two
antibiotics if the person has been feeling sick. Otherwise, below are
other options for treatments.
Intestinal
Usually nitroimidazole derivatives
(such as metronidazole) are used, because they are highly effective against the
trophozoite form of the amoeba. Since they have little effect on amoeba cysts, usually this
treatment is followed by an agent (such as paromomycin or diloxanide furoate)
that acts on the organism in the lumen.
Liver abscess:
In addition to
targeting organisms in solid tissue, primarily with drugs like metronidazole and chloroquine, treatment of
liver abscess must include agents that act in the lumen of the intestine (as in
the preceding paragraph) to avoid re-invasion. Surgical drainage is usually not
necessary, except when rupture is imminent.
People without
symptoms: For people without symptoms (otherwise known as asymptomatic
carriers), non-endemic areas should be treated by paromomycin; other
treatments include diloxanide
furoate, and iodoquinol. There have been problems with the use of
iodoquinol and iodochlorhydroxyquin, so their use is not recommended.
Diloxanide furoate can also be used by mildly symptomatic persons who are just
passing cysts.
|
Genus
and |
Entamoeba
histolytica |
|
Etiologic
agent of: |
Amoebiasis; amoebic
dysentery;
extraintestinal amoebiasis, usually amoebic liver abscess; "anchovy
sauce"); amoeba cutis; amoebic lung abscess ("liver-colored
sputum") |
|
Infective
stage |
Tetranucleated
cyst (having 2–4 nuclei) |
|
Definitive
host |
Human |
|
Portal
of entry |
Mouth |
|
Mode
of transmission |
Ingestion
of mature cyst through contaminated food or water |
|
Habitat |
Colon
and cecum |
|
Pathogenic
stage |
|
|
Locomotive
apparatus |
Pseudopodia
("false foot”") |
|
Motility |
Active,
progressive and directional |
|
Nucleus |
'Ring
and dot' appearance: peripheral chromatin and central karyosome |
|
Mode
of reproduction |
Binary
fission |
|
Pathogenesis |
Lytic
necrosis (it looks like “flask-shaped” holes in Gastrointestinal tract
sections (GIT) |
|
Type
of encystment |
Protective
and Reproductive |
|
Lab
diagnosis |
Most
common is direct fecal smear (DFS) and staining (but does not allow
identification to species level); enzyme
immunoassay (EIA);
indirect hemagglutination (IHA); Antigen detection – monoclonal
antibody; PCR for species identification.
Sometimes only the use of a fixative (formalin) is effective in detecting
cysts. Culture: From faecal samples – Robinson's medium, Jones' medium |
|
Treatment |
Metronidazole for
the invasive trophozoites PLUS a lumenal amoebicide for those still in the
intestine. Paromomycin (Humatin)
is the luminal drug of choice, since Diloxanide
furoate (Furamide)
is not commercially available in the United States or Canada (being available
only from the Centers for Disease Control and Prevention). A direct
comparison of efficacy showed that Paromomycin had a higher cure rat. (Humatin) should be used with caution in
patients with colitis, as it is both nephrotoxic and ototoxic. Absorption
through the damaged wall of the intestinal tract can result in permanent
hearing loss and kidney damage. Recommended dosage: metronidazole 750 mg
three times a day orally, for 5 to 10 days followed by paromomycin
30 mg/kg/day orally in 3 equal doses for 5 to 10 days or Diloxanide
furoate 500 mg 3 times a day orally for 10 days, to eradicate lumenal
amoebae and prevent relapse. |
|
Trophozoite
stage |
|
|
Pathognomonic/diagnostic
feature |
Ingested
RBC; distinctive nucleus |
|
Cyst
Stage |
|
|
Chromatoidal
body |
'Cigar'
shaped bodies (made up of crystalline ribosomes) |
|
Number
of nuclei |
1
in early stages, 4 when mature |
|
Pathognomonic/diagnostic
feature |
'Ring
and dot' nucleus and chromatoid bodies |
Meiosis
In sexually
reproducing eukaryotes, homologous recombination (HR) ordinarily occurs
during meiosis. The
meiosis-specific recombinase, Dmc1, is required for efficient
meiotic HR, and Dmc1 is expressed in E. histolytica. The purified
Dmc1 from. histolytica forms presynaptic filaments
and catalyzes ATP-dependent homologous DNA pairing and DNA strand exchange over
at least several thousand base pairs. The DNA pairing and strand
exchange reactions are enhanced by the eukaryotic meiosis-specific
recombination accessory factor (heterodimer) Hop2-Mnd1. These processes are central to meiotic
recombination, suggesting that E. histolytica undergoes
meiosis.
Several other
genes involved in both mitotic and meiotic HR are also present in E.
histolytica. HR is enhanced under stressful growth conditions (serum
starvation) concomitant with the up-regulation of HR-related genes.
Also, UV irradiation induces DNA damage in E.
histolytica trophozoites and activates the
recombinational DNA repair pathway. In particular, expression of the Rad51 protein (a recombinase) is increased
about 15-fold by UV treatment.
Jan Ricks
Jennings, MHA, LFACHE
Senior Consultant
Senior Management
Resources. LLC
412.913.0636 Cell
724.733.0509
Office
JanJenningsBlog.BlogSpot.com
March 12, 2023
This article was
published on March 12, 2023. Born on
March 12, 1945 was the notorious gangster Salvatore "Sammy the
Bull" Gravano. He is an
American former mobster who
became underboss of the Gambino crime family. Gravano played a
major role in prosecuting John Gotti, the crime
family's boss, by agreeing to testify as a government witness against him
and other mobsters in a deal in which he confessed to involvement in 19
murders.














No comments:
Post a Comment