Leprosy
Sad fac of Leprosy
Leprosy, also known as Hansen's disease (HD), is
a long-term infection by the bacteria Mycobacterium leprae or Mycobacterium
lepromatosis. Infection
can lead to damage of the nerves, respiratory tract, skin, and eyes. This nerve damage may result in a lack of
ability to feel pain, which can lead to the loss of parts of a person's extremities from repeated injuries or infection through unnoticed
wounds. An infected
person may also experience muscle weakness and poor eyesight. Leprosy
symptoms may begin within one year, but, for some people, symptoms may take 20
years or more to occur.
Leprosy is spread between people, although extensive
contact is necessary. Leprosy has a low pathogenicity, and 95% of people who contract M. leprae do not
develop the disease. Spread is thought to occur through a cough or contact
with fluid from the nose of a person infected by leprosy. Genetic
factors and immune function play a role in how easily a person catches the
disease. Leprosy does not spread during pregnancy to the
unborn child or through sexual contact. Leprosy occurs more commonly among people living in
poverty. There are two main types of the disease
paucibacillary and multibacillary, which differ in the number of bacteria
present. A person with paucibacillary disease has five or
fewer poorly-pigmented, numb skin patches, while a person with multibacillary
disease has more than five skin patches. The diagnosis is confirmed by finding acid-fast bacilli in a biopsy of the skin.
Leprosy is curable with multidrug therapy. Treatment
of paucibacillary leprosy is with the medications dapsone, rifampicin, and clofazimine for six months. Treatment for multibacillary leprosy uses the same
medications for 12 months. A number of other antibiotics may also be used. These
treatments are provided free of charge by the World Health
Organization.
Leprosy is not highly contagious. People
with leprosy can live with their families and go to school and work. In the
1980s, there were 5.2 million cases globally but they went down to less
than 0.2 million by 2020 ] Most
new cases occur in 14 countries, with India accounting for more than half. In
the 20 years from 1994 to 2014, 16 million people worldwide were cured of
leprosy. About 200 cases per year are reported in
the United States. Separating people
affected by leprosy by placing them in leper colonies still occurs in some areas of India, China, the
African continent, and Thailand.
· Leprosy has affected humanity for thousands of
years. The disease takes its name from the Greek word λέπρᾱ (léprā),
from λεπῐ́ς (lepís; 'scale'), while the term "Hansen's
disease" is named after the Norwegian physician Gerhard Armauer Hansen. Leprosy has historically been associated with social stigma, which continues to be a barrier to self-reporting and
early treatment. Some consider the word leper offensive,
preferring the phrase "person affected with leprosy". Leprosy
is classified as a Wneglected tropical disease. world Leprosy Day was started in 1954 to draw awareness to those
affected by leprosy.
Signs and symptoms
Common symptoms present in the different types of leprosy
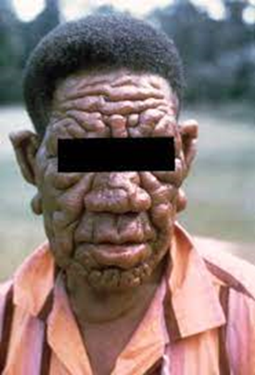
include a runny nose; dry scalp; eye problems; skin lesions; muscle weakness; reddish
skin; smooth, shiny, diffuse thickening of facial skin, ear, and hand; loss of
sensation in fingers and toes; thickening of peripheral nerves; a flat nose
from destruction of nasal cartilage; and changes in phonation and other aspects of speech production. In
addition, atrophy of the testes and impotence may occur.
Leprosy can affect people in different ways. The
average incubation period is five years. People may begin to notice symptoms within the first
year or up to 20 years after infection. The first noticeable sign of
leprosy is often the development of pale or pink colored patches of skin that
may be insensitive to temperature or pain. Patches of discolored skin are sometimes accompanied
or preceded by nerve problems including numbness or tenderness in the hands or
feet. Secondary infections (additional bacterial or viral infections) can result
in tissue loss, causing fingers and toes to become shortened and deformed, as
cartilage is absorbed into the body. A person's immune response differs depending on the
form of leprosy.
Approximately 30% of people affected with leprosy
experience nerve damage. The nerve damage sustained is reversible when treated
early, but becomes permanent when appropriate treatment is delayed by several
months. Damage to nerves may cause loss of muscle function, leading to
paralysis. It may also lead to sensation abnormalities or numbness, which may lead additional infections,
ulcerations, and joint deformities.
Paucibacillary
leprosy (PB): Pale skin patch with loss of sensation
·
Skin lesions
on the thigh of a person with leprosy
·
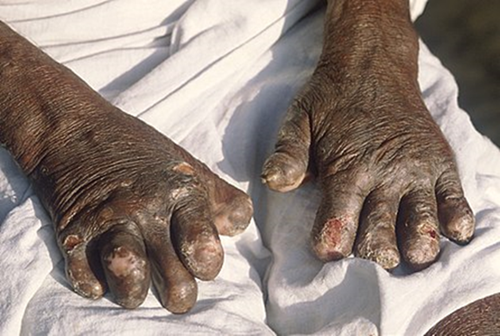
Hands
deformed by leprosy
Cause
M. leprae and M.
lepromatosis
M. leprae,
one of the causative agents of leprosy: As an acid-fast bacterium, M. leprae appears red
when a Ziehl–Nilsen stain is used.
M. leprae and M. lepromatosis are the
mycobacteria that cause leprosy.] M. lepromatosis is
a relatively newly identified mycobacterium isolated from a fatal case of diffuse
lepromatous leprosy in
2008. M. lepromatosis is
indistinguishable clinically from M. leprae.
M. leprae is an intracellular, acid-fast bacterium that is aerobic and rod-shaped. M. leprae is
surrounded by the waxy cell envelope coating characteristic of the genus Mycobacterium.
Genetically, M. leprae and M.
lepromatosis lack the genes that are necessary for independent growth. M.
leprae and M. lepromatosis are obligate intracellular pathogens, and cannot be grown (cultured) in the laboratory.] The
inability to culture M. leprae and M. lepromatosis has
resulted in a difficulty definitively identifying the bacterial organism under
a strict interpretation of Koch's postulates.
While the causative organisms have to date been impossible
to culture in vitro, it has been possible to grow them in animals
such as mice and armadillos.
Naturally occurring infection has been reported in nonhuman
primates (including the African chimpanzee, the sooty mangabey, and the cynomolgus macaque), armadillos, and red squirrels. Multilocus sequence typing of the armadillo M. leprae strains
suggests that they were of human origin for at most a few hundred years. Thus, it is suspected that
armadillos first acquired the organism incidentally from early American
explorers.[41] This
incidental transmission was sustained in the armadillo population, and it may
be transmitted back to humans, making leprosy a zoonotic disease (spread between humans and animals).
Red squirrels (Sciurus vulgaris), a threatened species in Great Britain, were found to
carry leprosy in November 2016. It has been suggested that the
trade in red squirrel fur, highly prized in the medieval period and intensively
traded, may have been responsible for the leprosy epidemic in medieval
Europe. A pre-Norman era skull excavated in Hoxne,
Suffolk, in 2017 was found to carry DNA from
a strain of Mycobacterium leprae, which closely matched the
strain carried by modern red squirrels on Brownsea Island,
UK.
Risk factors
The greatest risk factor for developing leprosy is contact
with another person infected by leprosy. People who are
exposed to a person who has leprosy are 5–8 times more likely to develop
leprosy than members of the general population. Leprosy also occurs more
commonly among those living in poverty. Not all people who are infected with M. leprae develop
symptoms.
Conditions that reduce immune function, such as
malnutrition, other illnesses, or genetic mutations, may increase the risk of
developing leprosy. Infection with HIV does not appear to increase the
risk of developing leprosy. Certain genetic factors in the
person exposed have been associated with developing lepromatous or tuberculoid
leprosy.
Transmission
Transmission of leprosy occurs during close contact with
those who are infected. Transmission of leprosy is through the upper respiratory tract. Older research suggested the skin as the main route
of transmission, but recent research has increasingly favored the respiratory
route. Transmission occurs through inhalation of bacilli present in
upper airway secretion.
Leprosy is not sexually transmitted and is not spread
through pregnancy to the unborn child. The majority (95%) of people who
are exposed to M. leprae do not develop leprosy; casual
contact such as shaking hands and sitting next to someone with leprosy does not
lead to transmission. People are considered non-infectious 72 hours after
starting appropriate multi-drug therapy.
Two exit routes of M. leprae from the human body
often described are the skin and the nasal mucosa, although their relative
importance is not clear. Lepromatous cases show large numbers of organisms deep
in the dermis, but whether they reach the skin surface in sufficient
numbers is doubtful.
Leprosy
may also be transmitted to humans by armadillos, although the mechanism is. not fully understood.
Genetics
Not
all people who are infected or exposed to M. leprae develop leprosy,
and genetic factors are suspected to play a role in susceptibility to an
infection. Cases of leprosy often cluster in families and
several genetic variants have been identified. In many
people who are exposed, the immune system is able to eliminate the leprosy
bacteria during the early infection stage before severe symptoms develop. A
genetic defect in cell-mediated
immunity may
cause a person to be susceptible to develop leprosy symptoms after exposure to
the bacteria. The region of DNA responsible for this variability is also involved
in Parkinson's disease, giving rise to current speculation that the two disorders
may be linked at the biochemical level.
Mechanics
Most leprosy complications are the result of nerve damage.
The nerve damage occurs from direct invasion by the M. leprae bacteria
and a person's immune response resulting in inflammation. The
molecular mechanism underlying how M. leprae produces the
symptoms of leprosy is not clear,[15] but M.
leprae has been shown to bind to Schwann cells, which may lead to nerve injury including demyelination and a loss of nerve function (specifically a loss
of axonal conductance). Numerous molecular mechanisms have been associated
with this nerve damage including the presence of a laminin-binding protein and the glycoconjugate (PGL-1) on the surface
of M. leprae that can bind to laminin on peripheral nerves.
As part of the human immune response, white blood cell-derived macrophages may engulf M. leprae by phagocytosis
In the initial stages, small sensory and autonomic nerve fibers in the skin of a person with leprosy are
damaged. This damage usually results in hair loss to the area, a
loss of the ability to sweat, and numbness (decreased ability to detect
sensations such as temperature and touch). Further peripheral nerve damage may
result in skin dryness, more numbness, and muscle weaknesses or paralysis in
the area affected. The skin can crack and if the skin injuries
are not carefully cared for,
there is a risk for a secondary infection that can lead to more severe damage.
Diagnosis
Testing
for loss of sensation with monofilament
In
countries where people are frequently infected, a person is considered to have
leprosy if they have one of the following two signs:
· Skin lesion consistent with leprosy and with definite
sensory loss.
· Positive skin smears.
Skin lesions can be single or many, and usually hypopigmented, although occasionally reddish or copper-colored. The
lesions may be flat (macules), raised (papules), or solid elevated areas (nodular). Experiencing sensory loss at the skin lesion is a
feature that can help determine if the lesion is caused by leprosy or by
another disorder such as tinea versicolor. Thickened nerves are associated with leprosy
and can be accompanied by loss of sensation or muscle weakness, but muscle
weakness without the characteristic skin lesion and sensory loss is not
considered a reliable sign of leprosy.
In some cases, acid-fast leprosy bacilli in skin smears are considered diagnostic; however,
the diagnosis is typically made without laboratory tests, based on
symptoms. If a person has a new leprosy diagnosis and already
has a visible disability caused by leprosy, the diagnosis is considered late.
In countries or areas where leprosy is uncommon, such as
the United States, diagnosis of leprosy is often delayed because healthcare
providers are unaware of leprosy and its symptoms. Early
diagnosis and treatment prevent nerve involvement, the hallmark of leprosy, and
the disability it causes.
There is no recommended test to diagnose latent leprosy in
people without symptoms.T Few people with latent leprosy test positive for
anti PGL-1. The presence of M. leprae bacterial DNA can be identified using a polymerase
chain reaction (PCR)-based
technique. This molecular test alone is not sufficient to
diagnose a person, but this approach may be used to identify someone who is at
high risk of developing or transmitting leprosy such as those with few lesions
or an atypical clinical presentation.
Classification
Several
different approaches for classifying leprosy exist. There are similarities
between the classification approaches.
· The World Health Organization system distinguishes
"paucibacillary" and "multibacillary" based upon the
proliferation of bacteria. ("pauci-" refers to a low quantity.)
· The Ridley-Jopling scale provides five gradations.
· The ICD-10,
though developed by the WHO, uses Ridley-Jopling and not the WHO system. It
also adds an indeterminate ("I") entry.
· In MeSH,
three groupings are used.
Complications
Leprosy may cause the victim to lose limbs and digits but
not directly. M. leprae attacks nerve endings and destroys the body’s ability
to feel pain and injury. Without feeling pain, people injure themselves on
fire, thorns, rocks, and even hot coffee cups. Injuries become infected and
result in tissue loss. Fingers, toes, and limbs become shortened and deformed
as the tissue is absorbed into the body.
Prevention
Early detection of the disease is important, since physical
and neurological damage may be irreversible even if cured. Medications
can decrease the risk of those living with people who have leprosy from acquiring
the disease and likely those with whom people with leprosy come into contact
outside the home. The WHO recommends that preventive
medicine be given to people who are in close contact with someone who has
leprosy.[10] The
suggested preventive treatment is a single dose of rifampicin (SDR) in adults
and children over 2 years old who do not already have leprosy or
tuberculosis. Preventive treatment is associated with a 57%
reduction in infections within 2 years and a 30% reduction in infections within
6 years.
The Bacillus
Calmette–Guérin (BCG) vaccine
offers a variable amount of protection against leprosy in addition to its
closely related target of tuberculosis. It appears to be 26% to 41% effective (based
on controlled trials) and about 60% effective based on observational studies
with two doses possibly working better than one. The WHO concluded
in 2018 that the BCG vaccine at birth reduces leprosy risk and is recommended
in countries with high incidence of TB and people who have
leprosy. People living in the same home as a person with
leprosy are suggested to take a BCG booster which may improve their immunity by
56%. Development of a more effective vaccine is ongoing
A novel vaccine called LepVax entered clinical trials in
2017 with the first encouraging results reported on 24 participants published
in 2020. If successful, this would be the first
leprosy-specific vaccine available.
Treatment
Anti-leprosy
medication
A number of leprostatic agents are available for treatment. A three-drug regimen
of rifampicin, dapsone and clofazimine is recommended for all people with leprosy, for six
months for paucibacillary leprosy and 12 months for multibacillary leprosy.
Multidrug therapy (MDT) remains highly effective, and
people are no longer infectious after the first monthly dose. It is
safe and easy to use under field conditions because of its presentation in
calendar blister packs. Post-treatment relapse rates remain
low. Resistance has been reported in several countries,
although the number of cases is small. People with
rifampicin-resistant leprosy may be treated with second line drugs such
as fluoroquinolones, minocycline, or clarithromycin, but the treatment duration is 24 months because of their
lower bactericidal activity.[90] Evidence
on the potential benefits and harms of alternative regimens for drug-resistant
leprosy is not yet available.
Skin changes
For people with nerve damage, protective footwear may help
prevent ulcers and secondary infection. Canvas shoes may be
better than PVC boots. There may be no difference between double
rocker shoes and below-knee plaster.
Topical ketanserin seems
to have a better effect on ulcer healing than clioquinol cream or zinc paste, but the evidence for this is
weak. Phenytoin applied to the skin improves skin changes to a
greater degree when compared to saline dressings.
Outcomes
Although leprosy has been curable since the mid-20th
century, left untreated it can cause permanent physical impairments and damage
to a person's nerves, skin, eyes, and limbs. Despite leprosy not
being very infectious and having a low pathogenicity, there is still
significant stigma and prejudice associated with the
disease. Because of this stigma, leprosy can affect a person's
participation in social activities and may also affect the lives of their
family and friends. People with leprosy are also at a higher
risk for problems with their mental well-being. The social stigma
may contribute to problems obtaining employment, financial difficulties, and
social isolation. Efforts to reduce discrimination and
reduce the stigma surrounding leprosy may help improve outcomes for people with
leprosy
.
Epidemiology
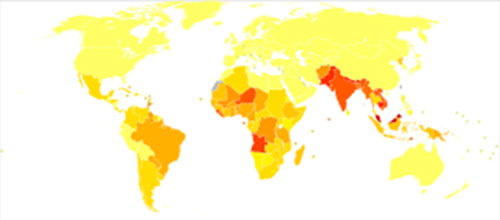
In 2018, there were 208,619 new cases of leprosy recorded,
a slight decrease from 2017. In 2015, 94% of the new leprosy cases were
confined to 14 countries. India reported the greatest number of new cases (60%
of reported cases), followed by Brazil (13%) and Indonesia (8%). Although the number of cases worldwide continues to
fall, there are parts of the world where leprosy is more common, including
Brazil, South Asia (India, Nepal, Bhutan), some parts of Africa (Tanzania,
Madagascar, Mozambique), and the western Pacific.[98] About
150 to 250 cases are diagnosed in the United States each year.
In the 1960s, there were tens of millions of leprosy cases recorded
when the bacteria started to develop resistance to dapsone, the most common treatment option at the time. International
(e.g., the WHO's "Global Strategy for Reducing Disease Burden Due to
Leprosy") and national (e.g., the International Federation of Anti-Leprosy
Associations) initiatives have reduced the total number and the number of new
cases of the disease.
Disease burden
The number of new leprosy cases is difficult to measure and
monitor because of leprosy's long incubation period, delays in diagnosis after
onset of the disease, and lack of medical care in affected areas. The
registered prevalence of the disease is used to determine disease burden. Registered prevalence is a useful proxy indicator of
the disease burden, as it reflects the number of active leprosy cases diagnosed
with the disease and receiving treatment with MDT at a given point in
time. The prevalence rate is defined as the number of cases registered for
MDT treatment among the population in which the cases have occurred, again at a
given point in time.
History
G. H. A.
Hansen, discoverer of M. leprae
Historical distribution
Using comparative genomics, in 2005, geneticists traced the origins and worldwide
distribution of leprosy from East Africa or the Near East along human migration
routes. They found four strains of M. leprae with specific
regional locations: ] Monot et
al. (2005) determined that leprosy originated in East Africa
or the Near East and traveled with humans along their migration routes,
including those of trade in goods and slaves. The four strains of M. leprae are
based in specific geographic regions where each predominantly occurs:
· strain 1 in Asia, the Pacific region, and East Africa;
· strain 2 in Ethiopia, Malawi, Nepal, north India, and New Caledonia;
· strain 3 in Europe, North Africa, and the Americas;
· strain 4 in West Africa and the Caribbean.
This confirms the spread of the disease along the
migration, colonization, and slave trade routes taken from East Africa to
India, West Africa to the New World, and from Africa into Europe and vice
versa.
Skeletal remains discovered in 2009 represent the oldest
documented evidence for leprosy, dating to the 2nd millennium BC. Located at Balathal, Rajasthan, in northwest India, the discoverers suggest
that, if the disease did migrate from Africa to India during the 3rd millennium BC "at a time when there was substantial
interaction among the Indus Civilization, Mesopotamia, and Egypt, there needs
to be additional skeletal and molecular evidence of leprosy in India and Africa
to confirm theAffrican origin of the disease." A proven human case
was verified by DNA taken from the shrouded remains of a man discovered by
researchers from the Hebrew
University of Jerusalem in
a tomb next to the Old City of Jerusalem, Israel, dated by radiocarbon methods to the first half of the 1st
century.
Pick up here down from here.
The oldest strains of leprosy known from Europe are
from Great Chesterford in southeast England and dating back to AD 415–545.
These findings suggest a different path for the spread of leprosy, meaning it
may have originated in Western Eurasia. This study also indicates that there
were more strains in Europe at the time than previously determined.
Discovery and scientific progress
Literary attestation of leprosy is unclear because of the
ambiguity of many early sources, including the Indian Atharvaveda and Kausika Sutra, the Egyptian Ebers papyrus,
and the Hebrew Bible's various sections regarding signs of impurity (tzaraath). Clearly leprotic symptoms are
attested in the Indian doctor Sushruta's Compendium, originally dating to c. 600 BC but only
surviving in emended texts no earlier than the 5th century. They were
separately described by Hippocrates in 460 BC. However, Hansen's disease probably
did not exist in Greece or the Middle East before the Common Era. In 1846, Francis Adams produced The Seven Books of Paulus Aegineta which
included a commentary on all medical and surgical knowledge and descriptions
and remedies to do with leprosy from the Romans, Greeks, and Arabs.
Leprosy did not exist in the Americas before colonization by modern Europeans] nor
did it exist in Polynesia until the middle of the 19th century.
Distribution of leprosy around the world in 1891
The
causative agent of leprosy, M. leprae, was discovered by G. H. Armauer
Hansen in Norway in 1873, making it the
first bacterium to be identified as causing disease in humans.
Treatment
The
first effective treatment (promin) became available in the 1940s. In the
1950s, dapsone was introduced. The search for further effective
antileprosy drugs led to the use of clofazimine and rifampicin in the 1960s and 1970s.[121] Later,
Indian scientist Shantaram Yawalkar and his colleagues formulated a combined
therapy using rifampicin and dapsone, intended to mitigate bacterial
resistance. Multi-drug
therapy (MDT) combining all three drugs was first recommended by the WHO in 1981. These three antileprosy drugs are still used
in the standard MDT regimens.
Leprosy
was once believed to be highly contagious and was treated with mercury, as was syphilis, which was first described in 1530. Many early cases
thought to be leprosy could actually have been syphilis.
Resistance
has developed to initial treatment. Until the introduction of MDT in the early
1980s, leprosy could not be diagnosed and treated successfully within the
community.
Japan still has sanatoriums (although Japan's sanatoriums
no longer have active leprosy cases, nor are survivors held in them by law).
The
importance of the nasal mucosa in the transmission of M leprae was recognized as early as 1898 by Schäffer, in
particular, that of the ulcerated mucosa. The mechanism of plantar
ulceration in leprosy and its treatment was first described by Dr Ernest W Price.
Etymology
The
word "leprosy" comes from the Greek word "λέπος (lépos) –
skin" and "λεπερός (leperós) – scaly man".
Society and culture
Two lepers denied entrance to town, 14th
India
British India enacted the Leprosy Act of 1898 which
institutionalized those affected and segregated them by sex to prevent
reproduction. The act was difficult to enforce but was repealed in 1983 only
after multidrug therapy had become widely available. In 1983, the National
Leprosy Elimination Programme, previously the National Leprosy Control
Programme, changed its methods from surveillance to the treatment of people
with leprosy. India still accounts for over half of the global disease burden.
According to WHO, new cases in India during 2019 diminished to 114,451 patients
(57% of the world's total new cases). Until 2019, one could justify a
petition for divorce with the spouse's diagnosis of leprosy.
Treatment cost
Between 1995 and 1999, the WHO, with the aid of the Nippon Foundation, supplied all endemic countries with free multidrug
therapy in blister packs, channeled through ministries of health.
This free provision was extended in 2000 and again in 2005, 2010
and 2015 with donations by the multidrug therapy manufacturer Novartis through the WHO. In the latest agreement signed
between the company and the WHO in October 2015, the provision of free
multidrug therapy by the WHO to all endemic countries will run until the end of
2025. At the national level, nongovernment
organizations affiliated with the national
program will continue to be provided with an appropriate free supply of
multidrug therapy by the WHO.
Historical texts
Written accounts of leprosy date back thousands of years.
Various skin diseases translated as leprosy appear in the ancient Indian text,
the Atharava Veda,
by 600 BC. Another Indian text, the Manusmriti (200
BC), prohibited contact with those infected with the disease and made
marriage to a person infected with leprosy punishable.
The Hebraic root tsara or tsaraath (צָרַע, – tsaw-rah' – to be
struck with leprosy, to be leprous) and the Greek (λεπρός–lepros), are of
broader classification than the more narrow use of the term related to Hansen's
Disease. Any progressive skin disease (a whitening or
splotchy bleaching of the skin, raised manifestations of scales, scabs,
infections, rashes, etc....), as well as generalized molds and surface
discoloration of any clothing, leather, or discoloration on walls or surfaces
throughout homes all, came under the "law of leprosy" (Leviticus
14:54–57).[138] Ancient
sources such as the Talmud (Sifra
make clear that tzaraath refers to various
types of lesions or stains associated with ritual impurity and occurring on cloth, leather, or houses, as well
as skin. Traditional Judaism and Jewish rabbinical authorities, both historical
and modern, emphasize that the tsaraath of Leviticus is a
spiritual ailment with no direct relationship to Hansen's disease or phyical
contagions. The relation of tsaraath to "leprosy"
comes from translations of Hebrew Biblical texts into Greek and ensuing
misconceptions
All three Synoptic Gospels of the New Testament describe instances of Jesus healing people with
leprosy (Matthew 8:1–4, Mark 1:40–45,
and Luke 5:12–16).
The Bible's description of leprosy is congruous (if lacking detail) with the
symptoms of modern leprosy, but the relationship between this disease, tzaraath,
and Hansen's disease has been disputed.[140] The
biblical perception that people with leprosy were unclean can be found in a
passage from Leviticus 13: 44–46. While this text defines the leper as impure, it did not explicitly make a moral judgement on those
with leprosy.[141] Some Early Christians believed that those affected by leprosy were being
punished by God for sinful behavior. Moral associations have persisted
throughout history. Pope Gregory the Great (540–604) and Isidor of Seville (560–636) considered people with the disease to be
heretics.
Middle
Ages
Medieval leper
The social perception of leprosy in the general population
was in general mixed. On one hand, people feared getting infected with the
disease and thought of people suspected of leprosy to be unclean,
untrustworthy, and occasionally morally corrupt. On the other hand, Jesus'
interaction with lepers, the writing of church leaders and the Christian focus
on charitable works led to viewing the lepers as "chosen by God or
seeing the disease as a means of obtaining access to heaven.
Early medieval understanding of leprosy was influenced by
early Christian writers such as Gregory of Nazianzus and John Chrysostom, whose writings were later embraced by Byzantine and Latin
writers. Gregory, for example, did not only compose sermons
urging Christians to assist victims of the disease, but also condemned pagans
or Christians who justified rejecting lepers on the allegation that God had
sent them the disease to punish them. As cases of leprosy increased during
these years in the Eastern Roman Empire, becoming a major health issue, the ecclesiastic leaders
of the time discussed how to assist those affected as well as change the
attitude of society towards them. They also tried this by using the name
"Holy disease" instead of the commonly used "Elephant's
disease" (elephantiasis), implying that God did not create this disease to
punish people but to purify them for heaven.[146] Although
not always successful in persuading the public and a cure was never found by
Greek medicians, they created an environment where victims could get palliative care and were never expressly banned from society, as
sometimes happened in Western Europe. Theodore Balsamon, a 12th century jurist
in Constantinople, noted that lepers were allowed to enter the same
churches, cities and assemblies that healthy people attended.
As the disease became more prevalent in Western Europe in
the fifth century, first efforts to set up permanent institutions to house and
feed lepers. These efforts were, inclusively, the work of bishops in France at
the end of the sixth century, such as in Chalon-sur-Saône. The
increase in hospitals or leprosaria (sing. leprosarium) that treated people with leprosy
in the 12th and 13th century seems to indicate a rise in cases, possibly
in connection with the increase in urbanification as well as returning
crusaders from the Middle East.[145] France
alone had nearly 2,000 leprosaria during this period. Additionally
to the new leprosia, further steps were taken by secular and religious leaders
to prevent further spread of the disease. The third Lateran Council of 1179 required lepers to have their own priests and
churches and a 1346 edict by King Edward expelled lepers from city limits. Segregation from
mainstream society became common, and people with leprosy were often required
to wear clothing that identified them as such or carry a bell announcing their
presence. As
in the East, it was the Church who took care of the lepers due to the still
persisting moral stigma and who ran the leprosaria. Although
the leprosaria in Western Europe removed the sick from society, they were never
a place to quarantine them or from which they could not leave: lepers would go
beg for alms for the upkeep of the leprosaria or meet with their families.
19th
century
24-year-old
A man with leprosy (1886)
Norway
Norway
was the location of a progressive stance on leprosy tracking and treatment and
played an influential role in European understanding of the disease. In 1832,
Dr. JJ Hjort conducted the first leprosy survey, thus establishing a basis for
epidemiological surveys. Subsequent surveys resulted in the establishment of a
national leprosy registry to study the causes of leprosy and for tracking the
rate of infection.
Early
leprosy research throughout Europe was conducted by Norwegian scientists Daniel
Cornelius Danielssen and Carl Wilhelm Boeck. Their work resulted in the establishment of the National
Leprosy Research and Treatment Center. Danielssen and Boeck believed the cause
of leprosy transmission was hereditary. This stance was influential in
advocating for the isolation of those infected by sex to prevent reproduction.
Colonialism
and imperialism
Father Damien on his deathbed in 1889
Though leprosy in Europe was again on the decline by the 1860s,
Western countries embraced isolation treatment out of fear of the spread of
disease from developing countries, minimal understanding of bacteriology, lack of diagnostic
ability or knowledge of how contagious the disease was, and missionary
activity. Growing imperialism and pressures of the industrial
revolution resulted in a Western presence in countries where leprosy
was endemic, namely the British presence in India. Isolation treatment methods were observed by
Surgeon-Mayor Henry Vandyke Carter of the British Colony in India while visiting Norway,
and these methods were applied in India with the financial and logistical
assistance of religious missionaries. Colonial and religious influence and associated stigma
continued to be a major factor in the treatment and public perception of
leprosy in endemic developing countries until the mid-twentieth century.
20th
century United States
The
National Leprosarium at Carville, Louisiana, known in 1955 as the Louisiana Leper Home, was the only
leprosy hospital on the mainland United States. Leprosy patients from all over
the United States were sent to Carville in order to be kept in isolation away
from the public, as not much about leprosy transmission was known at the time
and stigma against those with leprosy was high (see Leprosy stigma). The Carville leprosarium was known for its innovations
in reconstructive surgery for those with leprosy. In 1941, 22 patients at
Carville underwent trials for a new drug called promin. The results were described as miraculous, and soon after
the success of promin came dapsone, a medicine even more effective in the fight against
leprosy.
Stigma
Despite
now effective treatment and education efforts, leprosy stigma continues to be
problematic in developing countries where the disease is common. Leprosy is
most common amongst impoverished populations where social stigma is likely to
be compounded by poverty. Fears of ostracism, loss of employment, or expulsion
from family and society may contribute to a delayed diagnosis and treatment.
Folk beliefs, lack of education, and religious connotations
of the disease continue to influence social perceptions of those affected in
many parts of the world. In Brazil, for example, folklore holds that leprosy is
a disease transmitted by dogs, or that it is associated with sexual
promiscuity, or that it is a punishment for sins or moral transgressions
(distinct from other diseases and misfortunes, which are in general thought of
as being according to the will of God).[158] Socioeconomic
factors also have a direct impact. Lower-class domestic workers who are often
employed by those in a higher socioeconomic class may find their employment in
jeopardy as physical manifestations of the disease become apparent. Skin
discoloration and darker pigmentation resulting from the disease also have
social repercussions.
In extreme cases in northern India, leprosy is equated with
an "untouchable" status that "often persists long after
individuals with leprosy have been cured of the disease, creating lifelong
prospects of divorce, eviction, loss of employment, and ostracism from family
and social networks."
·
Leprosy
·
A 26-year-old woman with leprous
lesions
·
A 13-year-old boy with severe leprosy
Public Policy
A goal of the World Health Organization is to
"eliminate leprosy" and in 2016 the organization launched
"Global Leprosy Strategy 2016–2020 Elimination of leprosy is defined
as "reducing the proportion of leprosy patients in the community to very
low levels, specifically to below one case per 10,000 population".] Diagnosis
and treatment with multidrug thepre effective, and a 45% decline in disease
burden has ocurred since multidrug therapy has become more widely
available. The organization emphasizes the importance of fully integrating
leprosy treatment into public health services, effective diagnosis and
treatment, and access to information. The approach includes supporting an
increase in health care professionals who understand the disease, and a
coordinated and renewed political commitment that includes coordination between
countries and improvements in the methodology for collecting and analysing
data.
Interventions
in the "Global Leprosy Strategy 2016–2020: Accelerating towards a
leprosy-free world":
· Early detection of cases focusing on children to reduce transmission
and disabilities.
· Enhanced healthcare services and improved access for people
who may be marginalized.
· For countries where leprosy is endemic, further
interventions include an improved screening of close contacts, improved treatment
regimens, and interventions to reduce stigma and discrimination against people
who have leprosy.
·
Community-based
interventions
In
some instances in India, community-based rehabilitation is embraced by local
governments and NGOs alike. Often, the identity cultivated by a community
environment is preferable to reintegration, and models of self-management and
collective agency independent of NGOs and government support have been
desirable and successful.
Notable cases
· Josephine Cafrine of Seychelles had leprosy from the age of 12 and kept a personal
journal that documented her struggles and suffering.[165][166][167] It
was published as an autobiography in 1923.
· Saint Damien De Veuster, a Roman Catholic priest from Belgium, himself eventually
contracting leprosy, ministered to lepers who had been placed under a
government-sanctioned medical quarantine on the island of Molokaʻi in the Kingdom of Hawaiʻi.
· Baldwin IV of
Jerusalem was a
Christian king of Latin Jerusalem who had leprosy.
· Josefina Guerrero was a Filipino spy during World War II, who used the Japanese fear of her leprosy to listen to
their battle plans and deliver the information to the American forces
under Douglas MacArthur.
· King Henry IV of England (reigned 1399 to 1413) possibly had leprosy.
· Vietn
· Ōtani Yoshitsugu, a Japanese daimyō
Leprosy in the media
· English author Graham Greene's novel A Burnt-Out Case is set in a leper colony in Belgian Congo. The story
is also predominantly about a disillusioned architect working with a doctor on
devising new cure and amenities for mutilated victims of lepers; the title,
too, refers to the condition of mutilation and disfigurement in the disease.
· Death metal band Death (metal band) has an album titled “Leprosy”.
· Forugh Farrokhzad made a 22-minute documentary about a leprosy colony
in Iran in 1962 titled The House Is
Black. The film
humanizes the people affected and opens by saying that "there is no
shortage of ugliness in the world, but by closing our eyes on ugliness, we will
intensify it."
· Moloka'i is a novel by Alan Brennert about a leper colony in Hawaii. This novel follows
the story of a seven-year-old girl taken from her family and put on the small
Hawaiian island of Molokai's leper settlement. Even though this is a fiction novel it
is based upon some very true and revealing incidents which occurred at this
Leprosy settlement.
· Jack London in 1909 published Koolau the Leper in his Tales of Hawai'i about
Molokai and people consigned to it circa 1893.
· The lead character in The
Chronicles of Thomas Covenant by Stephen R. Donaldson suffers from leprosy. His condition seems to be cured
by the magic of the fantasy land he finds himself in, but he resists believing
in its reality, for example, by continuing to perform a regular visual
surveillance of extremities as
a safety check. Donaldson gained experience with the disease as a young man in
India, where his father worked in a missionary for people with leprosy.
· In House of the
Dragon, the TV
adaptation of George R. R. Martin's Fire
and Blood, King Viserys
I Targaryen suffers from a debilitating disease where parts of
his body develop lesions and slowly rot away over time. Paddy Considine, the actor playing the role, explained on a podcast with Entertainment Weekly that Viserys suffers from "a form of
leprosy". Leprosy is not mentioned in the novel, where Viserys
instead suffers from various health issues relating to his obesity, including
infections and gout.
Infection of animals
Wild nine-banded
armadillos (Dasypus
novemcinctus) in south central United States often carry Mycobacterium leprae. This
is believed to be because armadillos have a low body temperature. Leprosy
lesions appear mainly in cooler body regions such as the skin and mucous
membranes of the upper
respiratory tract.
Because of armadillos' armor, skin lesions are hard to
see. Abrasions around the eyes, nose and feet are the most common
signs. Infected armadillos make up a large reservoir of M. leprae and
may be a source of infection for some humans in the United States or other
locations in the armadillos' home range. In armadillo leprosy, lesions do not
persist at the site of entry in animals, M. leprae multiply in macrophages at the site of inoculation and lymph nodes.
A recent outbreak in chimpanzees in West Africa is showing
that the bacteria can infect another species and also possibly have additional
rodent hosts.
Recent studies have demonstrated that the disease is
endemic in the UK red Eurasian squirrel population, with Mycobacterium
leprae and Mycobacterium lepromatosis appearing in different
populations. The Mycobacteria leprae strain discovered on Brownsea
Island is equated to one thought to have died out in the human
population in mediaeval times. Despite this, and speculation
regarding past transmission through trade in squirrel furs, there does not seem
to be a high risk of squirrel to human transmission from the wild population.
Although Leprosy continues to be diagnosed in immigrants to the UK, the last
known human case of leprosy arising in the UK was recorded over 200 years ago.
It has been shown that leprosy can reprogram cells in
mouse and armadillos models similarly as how Induced
pluripotent stem cells are
generated by the transcription
factors Myc, Oct3/4, Sox2 and Klf4.
Jan
Ricks Jennings, MHA , LFACHE
Senior
Consultant
Senior Management Resources, LLC
JanJenningsBlog.Blogspot.com
412.913.0636
Cell
724.733.0509
Office