Coccidioidomycosis or Valley
Coccidioidomycosis commonly known
as cocci
Valley fever, well as California
fever, desert rheumatism, or San Joaquin Valley fever,[4] is
a mammalian fungal disease caused
by Coccidioides immitis or Coccidioides posadasii.[5] Coccidioidomycosis
is endemic in certain parts
of the United States in Arizona, California, Nevada, New
Mexico, Texas, Utah,
and northern Mexico.[6]
C. immitis is
a dimorphic saprophytic fungus
that grows as a mycelium in
the soil and produces a spherule form
in the host organism.
It resides in the soil in
certain parts of the southwestern United States, most notably in California and Arizona. It
is also commonly found in northern Mexico, and parts of Central and South America. C.
immitis is dormant during long dry spells, then develops as a mold with
long filaments that break off into airborne spores when
it rains. The spores, known as arthroconidia,
are swept into the air by disruption of the soil, such as during construction,
farming, low-wind or singular dust events, or an earthquake. Windstorms
may also cause epidemics far from endemic areas. In December 1977, a windstorm
in an endemic area around Arvin,
California led to several hundred cases,
including deaths, in non-endemic areas hundreds of miles away.
Coccidioidomycosis is a common cause
of community-acquired pneumonia in
the endemic areas of the United States. Infections
usually occur due to inhalation of the arthroconidial spores after soil
disruption. The
disease is not contagious. In
some cases the infection may recur or become chronical
It was reported in 2022 that valley fever
had been increasing in California's Central Valley for years (1,000 cases
in Kern county in
2014, 3,000 in 2021); experts said that cases could rise across the American
west as the climate makes the landscape drier and hotter.
Classification
After Coccidioides infection,
coccidioidomycosis begins with Valley fever, which is its initial acute form.
Valley fever may progress to the chronic form and then to disseminated coccidioidomycosis.
Therefore, coccidioidomycosis may be divided into the following types:
·
Acute coccidioidomycosis, sometimes
described in literature as primary pulmonary coccidioidomycosis
·
Chronic coccidioidomycosis
·
Disseminated coccidioidomycosis,
which includes primary cutaneous coccidioidomycosis
Valley fever is not a contagious disease.
Signs and symptoms
A skin lesion
due to Coccidioides infection
An estimated 60% of people infected with
the fungi responsible for coccidioidomycosis have minimal to no symptoms, while
40% will have a range of possible clinical symptoms. Of
those who do develop symptoms, the primary infection is most often respiratory,
with symptoms resembling bronchitis or pneumonia that
resolve over a matter of a few weeks. In endemic regions, coccidioidomycosis is
responsible for 20% of cases of community-acquired pneumonia Notable coccidioidomycosis signs and symptoms
include a profound feeling of tiredness,
loss of smell and taste, fever,
cough, headaches, rash, muscle pain,
and joint pain.[3] Fatigue
capersist for many months after initial infection. The
classic triad of coccidioidomycosis known as "desert rheumatism"
includes the combination of fever, joint pains, and erythema nodosum.
A minority (3–5%) of infected individuals
do not recover from the initial acute infection and develop a chronic
infection. This can take the form of chronic lung infection or widespread
disseminated infection (affecting the tissues
lining the brain, soft
tissues, joints, and bone). Chronic infection is responsible for most of the
morbidity and mortality. Chronic fibrocavitary disease is manifested by cough
(sometimes productive of mucus), fevers, night sweats and weight loss Osteomyelitis,
including involvement of the spine, and meningitis may
occur months to years after initial infection. Severe lung disease may develop
in HIV-infected
persons.
Complications
Serious complications may occur in patients who have weakened immune systems, including severe pneumonia with respiratory failure and bronchopleural fistulas requiring resection, lung nodules, and possible disseminated form, where the infection spreads throughout the body. The disseminated form of coccidioidomycosis can devastate the body, causing skin ulcers, abscesses, bone lesions, swollen joints with severe pain, heart inflammation, urinary tract problems, and inflammation of the brain's lining, which can lead to death.
Cause
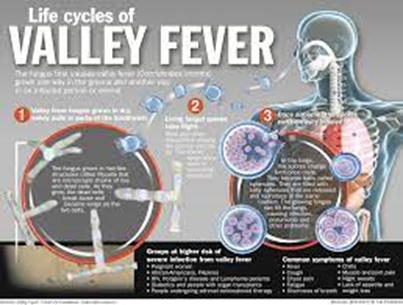
Life cycle
of Coccidioides
Both Coccidioides species
share the same asexual
life cycle, switching between saprobic (on
left) and parasitic (on
right) life stages.
Rain starts the cycle of initial growth
of the fungus in the soil. In
soil (and in agar media), Coccidioides exist
in filament form. It forms hyphae in
both horizontal and vertical directions. Over a prolonged dry period, cells
within hyphae degenerate to form alternating barrel-shaped cells (arthroconidia)
which are light in weight and carried by air currents. This happens when the
soil is disturbed, often by clearing trees, construction or farming. As the
population grows, so do all these activities, causing a potential cascade
effect. The more land that is cleared and the more arid the soil, the riper the
environment for Coccidioides. These spores can be easily
inhaled unknowingly. On reaching alveoli they
enlarge in size to become spherules, and internal septations develop.
This division of cells is made possible by the optimal temperature inside the
body. Septations
develop and form endospores within
the spherule. Rupture of spherules release these endospores, which in turn
repeat the cycle and spread the infection to adjacent tissues within the body
of the infected individual. Nodules can
form in lungs surrounding these spherules. When they rupture, they release
their contents into bronchi, forming thin-walled cavities. These cavities can
cause symptoms including characteristic chest pain, coughing up blood,
and persistent cough. In individuals with a weakened immune system, the
infection can spread through
the blood. The fungus can also, rarely,
enter the body through a break in the skin and cause infection.
Diagnosis
Coccidioidomycosis diagnosis relies on a
combination of an infected person's signs and symptoms, findings on
radiographic imaging, and laboratory results.[3] The
disease is commonly misdiagnosed as bacterial community-acquired
pneumonia.[3] The
fungal infection can be demonstrated by microscopic detection of diagnostic
cells in body fluids, exudates, sputum and biopsy tissue
by methods of Papanicolaou or Grocott's methenamine silver staining. These stains
can demonstrate spherules and surrounding inflammation.
With specific nucleotide primers, C.
immitis DNA can
be amplified by polymerase chain reaction (PCR).
It can also be detected in culture by morphological identification or by using
molecular probes that hybridize with C. immitis RNA. C.
immitis and C. posadasii cannot be distinguished on
cytology or by symptoms, but only by DNA PCR.
An indirect demonstration of fungal
infection can be achieved also by serologic analysis detecting fungal antigen or
host IgM or IgG antibody produced
against the fungus. The available tests include the tube-precipitin (TP)
assays, complement fixation assays,
and enzyme immunoassays.
TP antibody is not found in cerebrospinal
fluid (CSF). TP antibody is specific and
is used as a confirmatory test, whereas ELISA is sensitive and
thus used for initial
testing.[
If the meninges are affected, CSF will
show abnormally low glucose levels,
an increased level of protein, and lymphocytic pleocytosis.
Rarely, CSF eosinophilia is presen1
Chest X-rays rarely
demonstrate nodules or cavities in the lungs, but these images commonly
demonstrate lung opacification, associated with the lungs. Computed
tomography scans of the chest are more
sensitive than chest X-rays to detect these changes.
Prevention
Preventing Valley fever is challenging
because it is difficult to avoid breathing in the fungus should it be present;
however, the public health effect of the disease is essential to understand in
areas where the fungus is endemic. Enhancing surveillance of coccidioidomycosis
is key to preparedness in the medical field in addition to improving
diagnostics for early infections. Currently there are no completely effective
preventive measures available for people who live or travel through Valley
fever-endemic areas. Recommended preventive measures include avoiding airborne
dust or dirt, but this does not guarantee protection against infection. People
in certain occupations may be advised to wear face masks. The use
of air filtration indoors is also helpful, in addition to keeping skin injuries
clean and covered to avoid skin infection.
In 1998–2011, there were 111,117 cases of
coccidioidomycosis in the U.S. that were logged into the National
Notifiable Diseases Surveillance System (NNDSS). Since many U.S. states do not require
reporting of coccidioidomycosis, the actual numbers may be higher. The United
States' Centers for Disease Control and Prevention (CDC) called
the disease a "silent epidemic" and acknowledged that there is no
proven anticoccidioidal vaccine available. A
2001 cost-effectiveness analysis indicated
that a potential vaccine could improve health as well as reducing total health
care expenditures among infants, teens, and immigrant adults, and more modestly
improve health but increase total health care expenditures in older age groups.
Raising both surveillance and awareness
of the disease while medical researchers are developing a human vaccine can
positively contribute towards prevention efforts. Research
demonstrates that patients from endemic areas who are aware of the disease are
most likely to request diagnostic testing for
coccidioidomycosis. Presently, Meridian Bioscience manufactures the
so-called EIA test to diagnose the Valley fever, which however
is known for producing a fair quantity of false positives. Currently,
recommended prevention measures can include type-of-exposure-based respirator
protection for persons engaged in agriculture, construction and others working
outdoors in endemic areas. Dust control measures such as planting grass
and wetting the soil, and also limiting exposure to dust storms are advisable
for residential areas in endemic regions.
Treatment
Significant disease develops in fewer
than 5% of those infected and typically occurs in those with a weakened immune
system. Mild asymptomatic cases often do not require
any treatment. Those with severe symptoms may benefit from antifungal therapy,
which requires 3–6 months or more of treatment depending on the response to the
treatment. There is a lack of prospective studies that
examine optimal antifungal therapy for coccidioidomycosis.
On the whole, oral fluconazole and intravenous amphotericin B are
used in progressive or disseminated disease, or in immunocompromised
individuals. Amphotericin
B was originally the only available treatment, but
alternatives, including itraconazole and ketoconazole,
became available for milder disease. Fluconazole is the preferred medication for
coccidioidal meningitis, due to its penetration into CSF. Intrathecal or intraventricular amphotericin
B therapy is used if infection persists after fluconazole treatment. Itraconazole
is used for cases that involve treatment of infected person's bones and joints.
The antifungal medications posaconazole and voriconazole have
also been used to treat coccidioidomycosis. Because the symptoms of
coccidioidomycosis are similar to the common flu, pneumonia,
and other respiratory diseases, it is important for public health professionals
to be aware of the rise of coccidioidomycosis and the specifics of
diagnosis. Greyhound dogs
often get coccidioidomycosis; their treatment regimen involves 6–12 months of
ketoconazole taken with food.
A particular severe case of meningitis
caused by valley fever initially received several incorrect diagnoses such as
sinus infections and cluster headaches. The patient became unable to work
during diagnosis and original search for treatments. Eventually the right
treatment was found—albeit with severe side effects—requiring four pills a day
and medication administered directly into the brain every 16 weeks.
Toxicity
Conventional amphotericin B
desoxycholate (AmB:
used since the 1950s as a primary agent) is known to be associated with
increased drug-induced nephrotoxicity impairing kidney function.
Other
formulations have been developed such as lipid-soluble formulations to mitigate
side-effects such as direct proximal and distal tubular cytotoxicity.
These include liposomal amphotericin B, amphotericin
B lipid complex such as Abelcet (brand) amphotericin B
phospholipid complex[35] also
as AmBisome Intravenous, or Amphotec
Intravenous (Generic; Amphotericin B Cholesteryl Sul), and amphotericin
B colloidal dispersion, all shown to exhibit a decrease in nephrotoxicity.
The latter was not as effective in one study as amphotericin B
desoxycholate which had a 50% murine (rat
and mouse) morbidity rate versus zero for the AmB colloidal dispersion.
The cost of the nephrotoxic AmB
deoxycholate, in 2015, for a patient of 70 kilograms (150 lb) at
1 mg/kg/day dosage, was approximately US$63.80,
compared to $1318.80 for 5 mg/kg/day of the less toxic liposomal AmB.
Epidemiology
Coccidioidomycosis is endemic to the
western hemisphere between 40°N and 40°S. The ecological niches are
characterized by hot summers and mild winters with an annual rainfall of
10–50 cm. The species are found in alkaline sandy soil,
typically 10–30 cm below the surface. In harmony with the mycelium life
cycle, incidence increases with periods of dryness after a rainy season; this
phenomenon, termed "grow and blow", refers to growth of the fungus in
wet weather, producing spores which are spread by the wind dur
North America
In the United States, immitis is
endemic to southern and central California with the highest presence in
the San
Joaquin Valley. C.
posadassi is most prevalent in Arizona, although it can be found in a
wider region spanning from Utah, New Mexico, Texas, and Nevada. An estimated
150,000 infections occur annually, with 25,000 new infections occurring every
year.[contradictory] The
incidence of coccidioidomycosis in the United States in 2011 (42.6 per 100,000)
was almost ten times higher than the incidence reported in 1998 (5.3 per
100,000). In area where it is most prevalent, the infection rate is 2-4%.
Incidence varies widely across the west
and southwest. In Arizona, for instance, in 2007, there were 3,450 cases
in Maricopa County, which in
2007 had an estimated population of 3,880,181 for
an incidence of approximately 1 in 1,125. In
contrast, though southern New Mexico is considered an endemic region, there
were 35 cases in the entire state in 2008 and 23 in 2007, in
a region that had an estimated 2008 population of 1,984,356, for
an incidence of approximately 1 in 56,695.
Infection rates vary greatly by county,
and although population density is important, so are other factors that have
not been proven yet. Greater construction activity may disturb spores in the
soil. In addition, the effect of altitude on fungi growth and morphology has
not been studied, and altitude can range from sea level to 10,000 feet or
higher across California, Arizona, Utah and New Mexico.[
In California from 2000 to 2007, there
were 16,970 reported cases (5.9 per 100,000 people) and 752 deaths of the 8,657
people hospitalized. The highest incidence was in the San Joaquin Valley with
76% of the 16,970 cases (12,855) occurring in the area.[45] Following
the 1994 Northridge earthquake,
there was a sudden increase of cases in the areas affected by the quake, at a
pace of over 10 times baseline.
There was an outbreak in the summer of
2001 in Colorado, away from where the disease was considered endemic. A group
of archeologists visited Dinosaur National Monument,
and eight members of the crew, along with two National Park Service workers
were diagnosed with Valley fever.
California state prisons, beginning in
1919, have been particularly affected by coccidioidomycosis. In 2005 and 2006,
the Pleasant
Valley State Prison near Coalinga and Avenal State Prison near Avenal on
the western side of the San Joaquin Valley had
the highest incidence in 2005, of at least 3,000 per 100,000. The receiver appointed
in Plata v. Schwarzenegger issued
an order in May 2013 requiring relocation of vulnerable populations in those
prisons. The incidence rate has been increasing, with rates as high as 7%
during 2006–2010. The cost of care and treatment is $23 million in California
prisons. A lawsuit was filed against the state in 2014 on behalf of 58 inmates
stating that the Avenal and Pleasant valley state prisons did not take
necessary steps to prevent infections.
Population
risk factore
There are several populations that have a
higher risk for contracting coccidioidomycosis and developing the advanced
disseminated version of the disease. Populations with exposure to the airborne
arthroconidia working in agriculture and construction have a higher risk.
Outbreaks have also been linked to earthquakes, windstorms and military
training exercises where the ground is disturbed.[40] Historically,
an infection is more likely to occur in males than females, although this could
be attributed to occupation rather than being sex-specific. Women
who are pregnant and immediately postpartum are at a high risk of infection and
dissemination. There is also an association between stage of pregnancy and
severity of the disease, with third trimester women being more likely to
develop dissemination. Presumably this is related to highly elevated hormonal
levels, which stimulate growth and maturation of spherules and subsequent
release of endospores. Certain
ethnic populations are more susceptible to disseminated coccidioidomycosis. The
risk of dissemination is 175 times greater in Filipinos and 10 times greater in
African Americans than non-Hispanic whites. Individuals
with a weakened immune system are also more susceptible to the disease. In
particular, individuals with HIV and
diseases that impair T-cell function.
Individuals with pre-existing conditions such as diabetes are also at a higher
risk. Age also affects the severity of the disease, with more than one-third of
deaths being in the 65-84 age group.
The first case of what was later named
coccidioidomycosis was described in 1892 in Buenos Aires by Alejandro Posadas,
a medical intern at the Hospital
de Clínicas "José
de San Martín". Posadas
established an infectious character of the disease after being able to transfer
it in laboratory conditions to lab animals. In
the U.S., Dr. E. Rixford, a physician from a San Francisco hospital, and T. C.
Gilchrist, a pathologist at Johns Hopkins Medical School, became early pioneers
of clinical studies of the infection. They
decided that the causative organism was a Coccidia-type protozoan and
named it Coccidioides immitis (resembling Coccidia,
not mild).
Dr. William Ophüls, a professor at
Stanford University Hospital (San Francisco), discoverer. that the causative agent of the disease that
was at first called Coccidioides infection and later
coccidioidomycosis[58] was
a fungal pathogen, and coccidioidomycosis was also distinguished from Histoplasmosis and Blastomycosis.
Further, Coccidioides immitis was identified as the culprit of
respiratory disorders previously called San Joaquin Valley fever, desert fever,
and Valley fever, and a serum precipitin test was developed by Charles E. Smith
that was able to detect an acute form of the infection. In retrospect, Smith
played a major role in both medical research and raising awareness about
coccidioidomycosis,[59] especially
when he became dean of the School of Public Health at the University of
California at Berkeley in 1951.
Coccidioides immitis was
considered by the United States during the 1950s and 1960s as a potential
biological weapon.
The
strain selected for investigation was designated with the military symbol OC,
and initial expectations were for its deployment as a human incapacitant.
Medical research suggested that OC might have had some lethal effects on the
populace, and Coccidioides immitis started to be classified by
the authorities as a threat to public health. However, Coccidioides
immitis was never weaponized to the public's knowledge, and most of
the military research in the mid-1960s was concentrated on developing a human
vaccine.[
Currently, it is not on the U.S.
Department of Health and Human Services' or Centers for
Disease Control and Prevention's list
of select
agents and toxins.
In 2002, Coccidioides posadasii was
identified as genetically distinct from Coccidioides immitis despite
their morphologic similarities and can also cause coccidioidomycosis.
Research
As of 2013, there is no vaccine available to prevent infection with Coccidioides immitis or Coccidioides posadasii, but efforts to develop such a vaccine are underway.
A dog with
coccidioidomycosis.
Valley fever is not contagious.
In dogs, the most common symptom of
coccidioidomycosis is a chronic cough, which can be dry or moist. Other
symptoms include fever (in approximately 50% of cases), weight loss, anorexia,
lethargy, and depression. The disease can disseminate throughout
the dog's body, most commonly causing osteomyelitis (infection
of the bone), which leads to lameness. Dissemination can cause other symptoms,
depending on which organs are infected. If the fungus infects the heart
or pericardium,
it can cause heart
failure and death.
In cats, symptoms may include skin
lesions, fever, and loss of appetite, with skin lesions being the most common.
Other species in which Valley fever has
been found include livestock such as cattle and horses; llamas; marine mammals,
including sea otters; zoo animals such as monkeys and apes, kangaroos, tigers,
etc.; and wildlife native to the geographic area where the fungus is found,
such as cougars, skunks, and javelinas.
In
Popular Culture
·
In the Season 1 episode of Bones called
"The Man in the
Fallout Shelter" the
entire lab is exposed to coccidioidomycosis through inhalation of bone dust.
Erroneously, the team is forced to quarantine in the lab on Christmas Eve to
prevent the disease from spreading to the public (in real life, the disease is
not contagious).
o
The lab is later exposed to it agai
o
n in the Season 2 episode "The Priest in
the Churchyard" from
contaminated graveyard soil but only receives a series of injections rather
than be forced to quarantine.[70]
·
Everything in Between,
a 2022 Australian feature film, contains references to coccidioidomycosis.
·
In Doctor House, season 3 episode 4
"Line in the Sand", a 17 years old patient has a Coccidioides
infection.
Jan Ricks Jennings, MHA, LFACHE
Senior
Consultant
Senior
Management Resources, LLC
JanJenningsBlog.Blogspot.com
February
12, 2023




No comments:
Post a Comment