Factors in
the Emergence of Infectious Diseases
Abstract
"Emerging" infectious diseases can be defined as
infections that have newly appeared in a population or have existed but are
rapidly increasing in incidence or geographic range. Among recent examples are
HIV/AIDS, hantavirus pulmonary syndrome, Lyme disease, and hemolytic uremic
syndrome (a foodborne infection caused by certain strains of Escherichia
coli). Specific factors precipitating disease emergence can be identified
in virtually all cases. These include ecological, environmental, or demographic
factors that place people at increased contact with a previously unfamiliar
microbe or its natural host or promote dissemination. These factors are
increasing in prevalence; this increase, together with the ongoing evolution of
viral and microbial variants and selection for drug resistance, suggests that
infections will continue to emerge and probably increase and emphasizes the
urgent need for effective surveillance and control.
Infectious diseases emerging
throughout history have included some of the most feared plagues of the past.
New infections continue to emerge today, while many of the old plagues are with
us still. These are global problems (William Foege, former CDC director now at
the Carter Center, terms them global infectious disease threats). As
demonstrated by influenza epidemics, under suitable circumstances, a new
infection first appearing anywhere in the world could traverse entire
continents within days or weeks.
We can define as emerging
infections that have newly appeared in the population or have existed but are
rapidly increasing in incidence or geographic range. Recent examples of
emerging diseases in various parts of the world include HIV/AIDS, classic
cholera in South America and Africa, cholera due to Vibrio cholerae O139,
Rift Valley fever, hantavirus pulmonary syndrome, Lyme disease; and hemolytic
uremic syndrome, a foodborne infection caused by certain strains of Escherichia
coli (in the United States, surety.
Although these occurrences may
appear inexplicable, rarely if ever do emerging infections appear without
reason. Specific factors responsible for disease emergence can be identified in
virtually all cases studied. known causes for a number of infections that
have emerged recently. It has been suggested that infectious disease emergence
can be viewed operationally as a two-step process: Introduction of the agent
into a new host population (whether the pathogen originated in the environment,
possibly in another species, or as a variant of an existing human infection),
followed by establishment and further dissemination within the new host
population. Whatever its origin, the infection emerges when it reaches a new
population. Factors that promote one or both of these steps will, therefore,
tend to precipitate disease emergence. Most emerging infections, and even
antibiotic-resistant strains of common bacterial pathogens, usually originate
in one geographic location and then disseminate to new places.
Regarding the introduction step,
the numerous examples of infections originating as zoonoses suggest that the
zoonotic pool introductions of infections from other species is an important
and potentially rich source of emerging diseases; periodic discoveries of new
zoonoses suggest that the zoonotic pool appears by no means exhausted. Once
introduced, an infection might then be disseminated through other factors,
although rapid course and high mortality combined with low transmissibility are
often limiting. However, even if a zoonotic agent is not able to spread readily
from person to person and establish itself, other factors (e.g., nosocomial
infection) might transmit the infection. Additionally, if the reservoir host or
vector becomes more widely disseminated, the microbe can appear in new places.
Bubonic plague transmitted by rodent fleas and rat borne hantavirus infections
are examples.
Most emerging infections appear
to be caused by pathogens already present in the environment, brought out of
obscurity or given a selective advantage by changing conditions and afforded an
opportunity to infect new host populations (on rare occasions, a new variant
may also evolve and cause a new disease. The process by which infectious agents
may transfer from animals to humans or disseminate from isolated groups into
new populations can be called microbial traffic. A number of activities increase microbial
traffic and as a result promote emergence and epidemics. In some cases,
including many of the most novel infections, the agents are zoonotic, crossing
from their natural hosts into the human population; because of the many
similarities, I include here vector-borne diseases. In other cases, pathogens
already present in geographically isolated populations are given an opportunity
to disseminate further. Surprisingly often, disease emergence is caused by
human actions, however inadvertently; natural causes, such as changes in
climate, can also at times be responsible. Although this discussion is confined
largely to human disease, similar considerations apply to emerging pathogens in
other species.
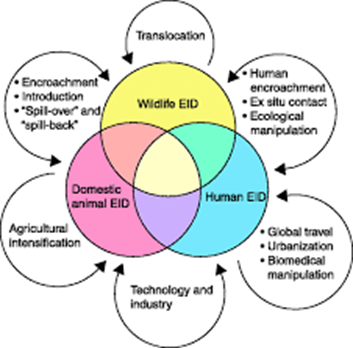
Any categorization of the
underlying factors responsible for emergence is, of course, somewhat arbitrary
but should be representative of the underlying processes that cause emergence.
I have essentially adopted the categories developed in the Institute of
Medicine report on emerging infections, with additional definitions from the
CDC emerging infections plan. Responsible factors include ecological changes,
such as those due to agricultural or economic development or to anomalies in
climate, human demographic changes and behavior, travel and commerce;
technology and industry, microbial adaptation and change, and breakdown of
public health measures. Each of these will be considered in turn. Examples of infections originating as zoonoses
suggest that the zoonotic pool introductions of infections from other species
is an important and potentially rich source of emerging diseases; periodic
discoveries of new zoonoses suggest that the zoonotic pool appears by no means
exhausted. Once introduced, an infection might then be disseminated through
other factors, although rapid course and high mortality combined with low
transmissibility are often limiting. However, even if a zoonotic agent is not
able to spread readily from person to person and establish itself, other
factors (e.g., nosocomial infection) might transmit the infection.
Additionally, if the reservoir host or vector becomes more widely disseminated,
the microbe can appear in new places. Bubonic plague transmitted by rodent
fleas and rat borne hantavirus infections are examples.
Ecological interactions can be
complex, with several factors often working together or in sequence. For
example, population movement from rural areas to cities can spread a
once-localized infection. The strain on infrastructure in the overcrowded and
rapidly growing cities may disrupt or slow public health measures, perhaps
allowing establishment of the newly introduced infection. Finally, the city may
also provide a gateway for further dissemination of the infection. Most
successful emerging infections, including HIV, cholera, and dengue, have
followed this route.
Consider HIV as an example.
Although the precise ancestry of HIV-1 is still uncertain, it appears to have
had a zoonotic origin. Ecological factors that would have allowed human
exposure to a natural host carrying the virus that was the precursor to HIV-1
were, therefore, instrumental in the introduction of the virus into humans.
This probably occurred in a rural area. A plausible scenario is suggested by
the identification of an HIV-2-infected man in a rural area of Liberia whose
virus strain resembled viruses isolated from the sooty mangabey monkey (an
animal widely hunted for food in rural areas and the putative source of HIV-2)
more closely than it did strains circulating in the city. Such findings suggest
that zoonotic introductions of this sort may occur on occasion in isolated
populations but may well go unnoticed so long as the recipients remain
isolated. But with increasing movement from rural areas to cities, such
isolation is increasingly rare. After its likely first move from a rural area
into a city, HIV-1 spread regionally along highways, then by long distance
routes, including air travel, to more distant places. This last step was
critical for HIV and facilitated today's global pandemic. Social changes that allowed
the virus to reach a larger population and to be transmitted despite its
relatively low natural transmissibility were instrumental in the success of the
virus in its newfound human host. For HIV, the long duration of infectivity
allowed this normally poorly transmissible virus many opportunities to be
transmitted and to take advantage of such factors as human behavior (sexual
transmission, intravenous drug use) and changing technology (early spread
through blood transfusions and blood products.
Ecological Changes and
Agricultural Development
Ecological changes, including
those due to agricultural or economic development, are among the most
frequently identified factors in emergence. They are especially frequent as
factors in outbreaks of previously unrecognized diseases with high
case-fatality rates, which often turn out to be zoonotic introductions.
Ecological factors usually precipitate emergence by placing people in contact
with a natural reservoir or host for an infection hitherto unfamiliar but usually
already present (often a zoonotic or arthropod-borne infection), either by
increasing proximity or, often, also by changing conditions so as to favor an
increased population of the microbe or its natural host. The emergence of Lyme
disease in the United States and Europe was probably due largely to
reforestation, which increased the population of deer and the deer tick, the
vector of Lyme disease. The movement of people into these areas placed a larger
population in close proximity to the vector.
Agricultural development, one of
the most common ways in which people alter and interpose themselves into the
environment, is often a factor. Hantan
virus, the cause of Korean hemorrhagic fever, causes over 100,000 cases a year
in China and has been known in Asia for centuries. The virus is a natural
infection of the field mouse Apodemus agrarius. The rodent
flourishes in rice fields; people usually contract the disease during the rice
harvest from contact with infected rodents. Junin virus, the cause of Argentine
hemorrhagic fever, is an unrelated virus with a history remarkably similar to
that of Hantan virus. Conversion of grassland to maize cultivation favored a
rodent that was the natural host for this virus, and human cases increased in
proportion with expansion of maize agriculture. Other examples, in addition to
those already known, are likely to appear as new areas are placed under
cultivation.
Perhaps most surprisingly,
pandemic influenza appears to have an agricultural origin, which is integrated
pig-duck farming in China. Strains causing the frequent annual or biennial
epidemics generally result from mutation , but pandemic influenza viruses do
not generally arise by this process. Instead, gene segments from two influenza
strains reassort to produce a new virus that can infect humans. Evidence
amassed by Webster, Scholtissek, and others, indicates that waterfowl, such as
ducks, are major reservoirs of influenza and that pigs can serve as mixing
vessels for new mammalian influenza strains. Pandemic influenza viruses have
generally come from China. Scholtissek and Naylor suggested that integrated
pig-duck agriculture, an extremely efficient food production system
traditionally practiced in certain parts of China for several centuries, puts
these two species in contact and provides a natural laboratory for making new
influenza recombinants. Webster has suggested that, with high-intensity
agriculture and movement of livestock across borders, suitable conditions may
now also be found in Europe.
Water is also frequently
associated with disease emergence. Infections transmitted by mosquitoes or other
arthropods, which include some of the most serious and widespread diseases, are
often stimulated by expansion of standing water, simply because many of the
mosquito vectors breed in water. There are many cases of diseases transmitted
by water-breeding vectors, most involving dams, water for irrigation, or stored
drinking water in cities. (See Changes in Human Demographics and Behavior for a
discussion of dengue.) The incidence of Japanese encephalitis, another
mosquito-borne disease that accounts for almost 30,000 human cases and
approximately 7,000 deaths annually in Asia, is closely associated with
flooding of fields for rice growing. Outbreaks of Rift Valley fever in some
parts of Africa have been associated with dam building as well as with periods of
heavy rainfall. In the outbreaks of Rift Valley fever in Mauritania in 1987,
the human cases occurred in villages near dams on the Senegal River. The same
effect has been documented with other infections that have aquatic hosts, such
as schistosomiasis.
Because humans are important
agents of ecological and environmental change, many of these factors are
anthropogenic. Of course, this is not always the case, and natural
environmental changes, such as climate or weather anomalies, can have the same
effect. The outbreak of hantavirus pulmonary syndrome in the southwestern
United States in 1993 is an example. It is likely that the virus has long been
present in mouse populations but an unusually mild and wet winter and spring in
that area led to an increased rodent population in the spring and summer and
thus to greater opportunities for people to come in contact with infected
rodents (and, hence, with the virus); it has been suggested that the weather
anomaly was due to large-scale climatic effects. The same causes may have been
responsible for outbreaks of hantaviral disease in Europe at approximately the
same time. With cholera, it has been suggested that certain organisms in marine
environments are natural reservoirs for cholera vibrios, and that large scale effects
on ocean currents may cause local increases in the reservoir organism with
consequent flare-ups of cholera.
Changes in Human Demographics
and Behavior
Human population movements or
upheavals, caused by migration or war, are often crucial factors in disease
emergence. In many parts of the world, economic conditions are encouraging the
mass movement of workers from rural areas to cities. The United Nations has
estimated that, largely as a result of continuing migration, by the year 2025,
65% of the world population (also expected to be larger in absolute numbers),
including 61% of the population in developing regions, will live in cities. As
discussed above for HIV, rural urbanization allows infections arising in
isolated rural areas, which may once have remained obscure and localized, to
reach larger populations. Once in a city, the newly introduced infection would
have the opportunity to spread locally among the population and could also
spread further along highways and interurban transport routes and by airplane.
HIV has been, and in Asia is becoming, the best known beneficiary of this
dynamic, but many other diseases, such as dengue, stand to benefit. The
frequency of the most severe form, dengue hemorrhagic fever, which is thought
to occur when a person is sequentially infected by two types of dengue virus,
is increasing as different dengue viruses have extended their range and now
overlap. Dengue hemorrhagic fever is now common in some cities in Asia, where
the high prevalence of infection is attributed to the proliferation of open
containers needed for water storage (which also provide breeding grounds for
the mosquito vector) as the population size exceeds the infrastructure. In
urban environments, rain-filled tires or plastic bottles are often breeding
grounds of choice for mosquito vectors. The resulting mosquito population boom
is complemented by the high human population density in such situations, increasing
the chances of stable transmission cycles between infected and susceptible
persons. Even in industrialized countries, e.g., the United States, infections
such as tuberculosis can spread through high-population density settings e.g.,
day care centers or prisons.
Human behavior can have important
effects on disease dissemination. The best known examples are sexually
transmitted diseases, and the ways in which such human behavior as sex or
intravenous drug use have contributed to the emergence of HIV are now well
known. Other factors responsible for disease emergence are influenced by a
variety of human actions, so human behavior in the broader sense is also very
important. Motivating appropriate individual behavior and constructive action,
both locally and in a larger scale, will be essential for controlling emerging
infections. Ironically, as AIDS prevention efforts have demonstrated, human
behavior remains one of the weakest links in our scientific knowledge.
International Travel and
Commerce
The dissemination of HIV through
travel has already been mentioned. In the past, an infection introduced into
people in a geographically isolated area might, on occasion, be brought to a
new place through travel, commerce, or war. Trade between Asia and Europe,
perhaps beginning with the silk route and continuing with the Crusades, brought
the rat and one of its infections, the bubonic plague, to Europe. Beginning in
the 16th and 17th centuries, ships bringing slaves from West Africa to the New
World also brought yellow fever and its mosquito vector, Aedes aegypti,
to the new territories. Similarly, smallpox escaped its Old World origins to
wreak new havoc in the New World. In the 19th century, cholera had similar
opportunities to spread from its probable origin in the Ganges plain to the
Middle East and, from there, to Europe and much of the remaining world. Each of
these infections had once been localized and took advantage of opportunities to
be carried to previously unfamiliar parts of the world.
Similar histories are being
repeated today, but opportunities in recent years have become far richer and
more numerous, reflecting the increasing volume, scope, and speed of traffic in
an increasingly mobile world. Rats have carried hantaviruses virtually worldwide. Aedes
albopictus (the Asian tiger mosquito) was introduced into the United
States, Brazil, and parts of Africa in shipments of used tires from Asia. Since
its introduction in 1982, this mosquito has established itself in at least 18
states of the United States and has acquired local viruses including Eastern
equine encephalomyelitis, a cause of serious disease. Another mosquito-borne
disease, malaria, is one of the most frequently imported diseases in
non-endemic-disease areas, and cases of airport malaria are occasionally
identified.
A classic bacterial disease,
cholera, recently entered both South America (for the first time this century)
and Africa. Molecular typing shows the South American isolates to be of the
current pandemic strain, supporting the suggestion that the organism was
introduced in contaminated bilge water from an Asian freighter. Other evidence
indicates that cholera was only one of many organisms to travel in ballast
water; dozens, perhaps hundreds, of species have been exchanged between distant
places through this means of transport alone. New bacterial strains, such as
the recently identified Vibrio cholerae O139, or an epidemic
strain of Neisseria meningitidis (also examples of microbial
adaptation and change) have disseminated rapidly along routes of trade and
travel, as have antibiotic-resistant bacteria.
High-volume rapid movement
characterizes not only travel, but also other industries in modern society. In
operations, including food production, that process or use products of
biological origin, modern production methods yield increased efficiency and reduced
costs but can increase the chances of accidental contamination and amplify the
effects of such contamination. The problem is further compounded by
globalization, allowing the opportunity to introduce agents from far away. A
pathogen present in some of the raw material may find its way into a large
batch of final product, as happened with the contamination of hamburger meat by E.
coli strains causing hemolytic uremic syndrome. In the United States
the implicated E. coli strains are serotype O157:H7; additional
serotypes have been identified in other countries. Bovine spongiform
encephalopathy (BSE), which emerged in Britain within the last few years, was
likely an interspecies transfer of scrapie from sheep to cattle that
occurred when changes in rendering processes led to incomplete inactivation of
scrapie agent in sheep byproducts fed to cattle.
The concentrating effects that
occur with blood and tissue products have inadvertently disseminated infections
unrecognized at the time, such as HIV and hepatitis B and C. Medical settings
are also at the front line of exposure to new diseases, and a number of
infections, including many emerging infections, have spread nosocomial in
health care settings. Among the numerous
examples, in the outbreaks of Ebola fever in Africa many of the secondary cases
were hospital acquired, most transmitted to other patients through contaminated
hypodermic apparatus, and some to the health care staff by contact.
Transmission of Lassa fever to health care workers has also been documented.
On the positive side, advances in
diagnostic technology can also lead to new recognition of agents that are
already widespread. When such agents are newly recognized, they may at first
often be labeled, in some cases incorrectly, as emerging infections. Human
herpesvirus 6 (HHV-6) was identified only a few years ago, but the virus
appears to be extremely widespread and has recently been implicated as the
cause of roseola (exanthem subitum), a very common childhood disease. Because roseola has been known since at least
1910, HHV-6 is likely to have been common for decades and probably much longer.
Another recent example is the bacterium Helicobacter pylori, a
probable cause of gastric ulcers and some cancers. We have lived with
these diseases for a long time without knowing their cause. Recognition of the
agent is often advantageous, offering new promise of controlling a previously
intractable disease, such as treating gastric ulcers with specific
antimicrobial therapy.
Microbial
Adaptation and Change
Microbes, like all other living
things, are constantly evolving. The emergence of antibiotic-resistant bacteria
as a result of the ubiquity of antimicrobials in the environment is an
evolutionary lesson on microbial adaptation, as well as a demonstration of the
power of natural selection. Selection for antibiotic-resistant bacteria and
drug-resistant parasites has become frequent, driven by the wide and sometimes
inappropriate use of antimicrobial drugs in a variety of applications. Pathogens
can also acquire new antibiotic resistance genes from other, often
nonpathogenic, species in the environment, selected or perhaps even driven by
the selection pressure of antibiotics.
Many viruses show a high mutation
rate and can rapidly evolve to yield new variants. A classic example is influenza. Regular
annual epidemics are caused by antigenic drift in a previously circulating
influenza strain. A change in an antigenic site of a surface protein, usually
the hemagglutinin (H) protein, allows the new variant to reinfect previously
infected persons because the altered antigen is not immediately recognized by
the immune system.
On rare occasions, perhaps more
often with nonviral pathogens than with viruses, the evolution of a new variant
may result in a new expression of disease. The epidemic of Brazilian purpuric
fever in 1990, associated with a newly emerged clonal variant of Hemophilus
influenzae, bio group aegyptius, may fall into this category.
It is possible, but not yet clear, that some recently described manifestations
of disease by group A Streptococcus, such as rapidly invasive
infection or necrotizing fasciitis, may also fall into this category.
Breakdown of Public Health
Measures and Deficiencies in Public Health Infrastructure
Classical public health and
sanitation measures have long served to minimize dissemination and human
exposure to many pathogens spread by traditional routes such as water or
preventable by immunization or vector control. The pathogens themselves often
still remain, albeit in reduced numbers, in reservoir hosts or in the
environment, or in small pockets of infection and, therefore, are often able to
take advantage of the opportunity to reemerge if there are breakdowns in
preventive measures.
Reemerging diseases are those, like
cholera, that were once decreasing but are now rapidly increasing again. These
are often conventionally understood and well recognized public health threats
for which (in most cases) previously active public health measures had been
allowed to lapse, a situation that unfortunately now applies all too often in
both developing countries and the inner cities of the industrialized world. The
appearance of reemerging diseases may, therefore, often be a sign of the
breakdown of public health measures and should be a warning against complacency
in the war against infectious diseases.
Cholera, for example, has
recently been raging in South America (for the first time in this
century) and Africa. The rapid spread of cholera in South America may have
been abetted by recent reductions in chlorine levels used to treat water
supplies. The success of cholera and other enteric diseases is often due to the
lack of a reliable water supply. These problems are more severe in developing
countries, but are not confined to these areas. The U.S. outbreak of
waterborne Cryptosporidium infection in Milwaukee, Wisconsin,
in the spring of 1993, with over 400,000 estimated cases, was in part due to a
nonfunctioning water filtration plant; similar deficiencies in water
purification have been found in other cities in the United States.
Dr. David Satcher has discussed
the history of infectious diseases and the many infections that, from the dawn
of history to the present, have traveled with the caravans and followed the
invading armies. The history of infectious diseases has been a history of
microbes on the march, often in our wake, and of microbes that have taken
advantage of the rich opportunities offered them to thrive, prosper, and
spread. And yet the historical processes that have given rise to the emergence
of new infections throughout history continue today with unabated force; in
fact, they are accelerating, because the conditions of modern life ensure that
the factors responsible for disease emergence are more prevalent than ever
before. Speed of travel and global reach are further borne out by studies
modeling the spread of influenza epidemics and HIV.
Humans are not powerless,
however, against this relentless march of microbes. Knowledge of the factors
underlying disease emergence can help focus resources on the key situations and
areas worldwide and develop more effective prevention strategies. If we are to
protect ourselves against emerging diseases, the essential first step is
effective global disease surveillance to give early warning of emerging
infections. This must be tied to incentives, such as national development, and
eventually be backed by a system for an appropriate rapid response. World
surveillance capabilities are critically deficient. Efforts, such as the CDC
plan, now under way in the United States and internationally to remedy this
situation are the essential first steps and deserve strong support. Research,
both basic and applied, will also be vital.
Jan Ricks Jennings, MHA, LFACHE
Senior Consultant
Senior
Management Resources, LLC
JanJenningsBlog.Blogspot.com
412.913.0636 Cell
724.733.0509 Office
December 11, 2022
P.S. Pope Leo the X was born on December 11, 1475. As popes go, he did not go down in history as the most successful leader of the Roman Catholic church. He borrowed and spent money without circumspection. He was incredibly opposed to the protestant reformation. He was a notable patron of the arts and had considerable success in the rebuilding of the Bassilica at the Vatican. He died at age 45.




No comments:
Post a Comment